About Us | Overview
TuHURA
Biosciences
We are a Phase 3 immuno-oncology company with three distinct technologies focused on the development of novel therapeutics designed to overcome primary and acquired resistance to cancer immunotherapies.
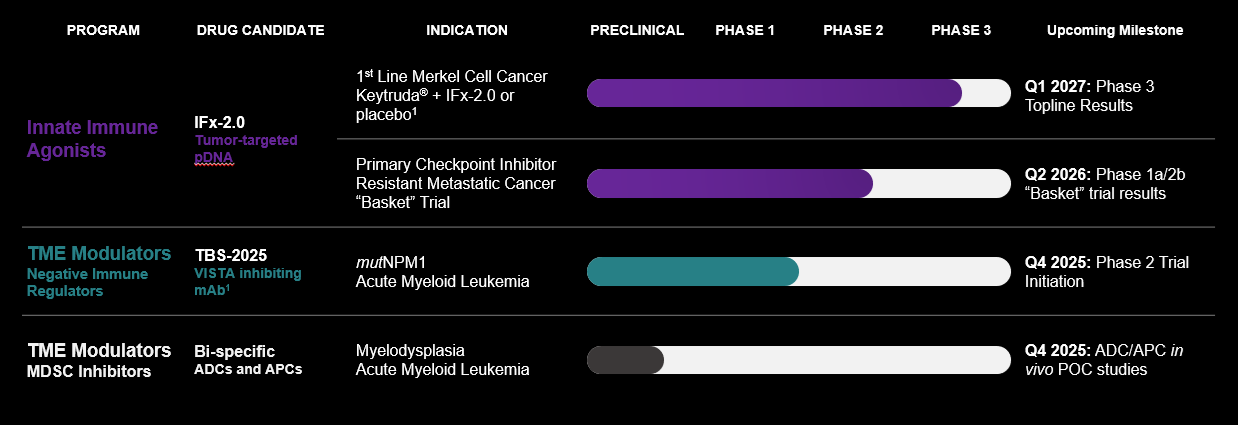
Our proprietary Immune FxTM technology platform, or IFx, is an innate immune agonist technology designed to “trick” the body’s immune system to attack tumor cells by making tumor cells look like bacteria. Our lead product candidate, IFx2.0, is an innate immune agonist designed to overcome primary resistance to checkpoint inhibitors. In June 2025, we initiated a single randomized placebo-controlled Phase 3 registration trial of IFx-2.0 administered as an adjunctive therapy to Keytruda® (pembrolizumab) in first line treatment for patients with advanced or metastatic Merkel cell carcinoma who are checkpoint inhibitor naïve utilizing the FDA’s accelerated approval pathway.
In addition to our IFx technology platform, we acquired the rights to TBS-2025, a novel VISTA-inhibiting monoclonal antibody formerly known as KVA1213, through our acquisition in June 2025 of Kineta, Inc. VISTA (otherwise referred to as V-domain Ig suppressor of T cell activation) is an immune checkpoint highly expressed on myeloid cells that is a strong driver of immunosuppression in the tumor microenvironment and a primary mechanism by which leukemic blasts escape immune recognition contributing to low response rates and high rates of recurrence in acute myeloid leukemia, or AML. Following our acquisition of Kineta, we are currently planning on investigating TBS-2025 in a randomized Phase 2 trial in combination with a menin inhibitor vs menin inhibitor alone in mutated NPM1 (mutNPM1) AML.
In addition to our IFx and TBS-2025 technologies, we are leveraging our Delta Opioid Receptor technology to develop tumor microenvironment modulators in the form of first-in-class bi-specific antibody-peptide conjugates (“APCs”) and antibody-drug conjugates (“ADCs”) targeting Myeloid Derived Suppressor Cells (“MDSCs”). Our APCs and ADCs are being developed to inhibit the immune-suppressing effects of MDSCs on the tumor microenvironment to prevent T cell exhaustion and acquired resistance to checkpoint inhibitors and cellular therapies.
Our Strategy
Our goal is to become a leading immuno-oncology company by developing novel therapeutics designed to overcome primary and acquired resistance to cancer immunotherapies, thereby broadening the impact of therapies such as checkpoint inhibitors. The key elements of this strategy include:
Pipeline
Your tooltip content goes here
Your tooltip content goes here
Your tooltip content goes here
